Pioneering Plasmid Manufacturing: A Time and Money-Saving Platform Making History
For more information, connect with our experts by clicking on the contact us tab below.
Zero Chrom Plasmid are available off-of-the-shelf in both PD and GMP grades
CoJourney has off-the-shelf plasmids available to purchase. These research-grade plasmids are available as per your needs from milligram to gram scale.
For more information on the quote on the off-the-shelf plasmids, click the tab below.
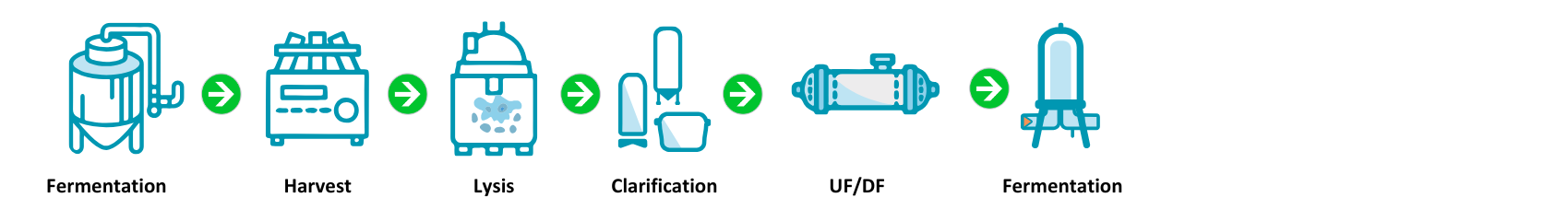
CoJourney’s revolutionary Zero Chrom Plasmid Platform was developed by process experts to transform plasmid manufacturing, enhancing quality and reducing costs.
CoJourney’s industry-leading platform is supported by 5 production lines that generate over 350+ batches per year.
Process Development
R&D Batch Fermentation
GMP Batch Fermentation
GMP Manufacturing Platform

CoJourney produces plasmids with high yield, high purity, low residual, and low endotoxin without the use of chromatography. However, optional anion exchange (AEX) or hydrophobic interaction chromatography (HIC) is available.
Plasmid Specifications
High quality plasmids are critical component of a growing number of advanced therapies. Plasmids can be packaged into viral vectors for cell and gene therapies, serve as templates for mRNA transcription, and are administered directly as vaccines or therapeutics.
Junhui Li
Junhui Li has over 20 years of experience in biologic drug development, manufacturing, and commercialization, with expertise in plasmid, lentiviral, and CAR-T cell manufacturing and quality control, mammalian cell platform development, and process development and GMP production of viral vectors. He previously worked for the international companies ICT and RAAS, where he led research and development, product manufacturing, and supported several IND submissions. Junhui holds both a Ph.D. in Biochemical Engineering and an MBA.
Guang Gao
Guang Gao is an FDA Master Reviewer with over 20 years of FDA regulatory review experience. Guang has reviewed over 800 applications (BLA, PMA, IND, IDE and 510(k)) and has directly contributed to the establishment of rules and regulations for the production of safe and effective medical devices.
Jerry Liu
Jerry Liu is an experienced business development and marketing leader, having served in various roles at Danaher BTG, Cytiva, GE Lifescience, PerkinElmer, and ThermoFisher. With a Doctorate in Business Administration, an EMBA, post-graduate diploma in Finance, and an M.Sc. in Bio-Chemistry, Jerry has a thorough understanding of the CGT market, and the requirements for development and production of high quality CGT products.
Lijun Wang
Lijun Wang has over 20 years of industry experience, with 16 years concentrated in gene therapy process development and GMP manufacturing. She led process development and GMP manufacturing for over 25 batches while employed at Brammer Bio. She also led downstream process development at Applied Genetic Technologies Corporation. Lijun has designed 6 facilities for the manufacture of high quality gene therapy products.